Study Finds Medication Eases
Side Effect of Cancer Treatment
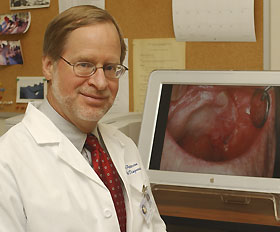 |
|
Dr. Douglas Peterson, a professor of oral diagnosis and associate director of the Carole and Ray Neag Comprehensive Cancer Center, has studied a medication to treat oral mucositis, a side effect of chemotherapy. |
|
Photo by Bill Hengstenberg
|
Painful mouth sores from chemotherapy used for cancer treatment have been significantly
reduced by a new medication that recently completed a Phase III
clinical study, according to Dr. Douglas Peterson, a professor of oral diagnosis
and associate director of the Carole and Ray Neag Comprehensive Cancer Center.
“The study medication reduced the incidence of the painful sores, known as oral mucositis, by 22 percent,” says Peterson, who was scientific advisor for the study sponsored by manufacturer Asegen Inc. of Princeton, N.J. He announced the results last summer at the annual meeting of the American Society of Clinical Oncology.
Oral mucositis afflicts about 400,000 cancer patients every year in the United States alone. The condition can seriously affect the quality of life of the patient. Not only does it interfere with nutrition during cancer treatment, it can pose a major risk of infection that spreads throughout the body in patients with low blood counts. The complications can result in serious illness or death.
“Oral mucositis can be one of the main reasons why patients suspend chemotherapy or head and neck radiation that may be saving or prolonging their lives,” says Peterson.
Some cancer patients who suffer from oral mucositis must be hospitalized and fed intravenously and given opiates or narcotics to control their mouth pain.
“Treatment for the mouth sores in the critically ill can cost as much as $40,000 for every patient,” Peterson says. “It can be a very expensive toxicity to manage.”
The oral mucositis study evaluated 2,084 women with breast cancer who received three cycles of anthracycline-based chemotherapy. During the first cycle of treatment, none of the women received the study medication, and nearly 16 percent, or 326 women, experienced clinically significant oral mucositis.
Those 326 women were then divided into two groups. One group received the medication during the second cycle of chemotherapy and placebo during the third cycle. The other group received placebo during the second cycle and the medication during the third cycle.
“We found that the medication significantly reduced the mouth sores during the chemotherapy cycle in which it was given,” says Peterson. “We also found a significant protective effect that carried over into the next cycle.
“The carryover benefit was a surprise,” he adds, “and represents an important new direction for the research and patient treatment, namely, consideration of pretreatment and longer-term treatment to prevent oral mucositis in patients receiving multiple cycles of chemotherapy.”
The drug (AES-14, Saforis) consists of a proprietary drug delivery system that enhances by up to 100-fold the absorption of a beneficial amino acid into the cells that make up the lining of the mouth. The amino acid occurs naturally in the body and is depleted in cancer patients undergoing chemotherapy. High levels of the amino acid help the lining of the mouth recover from and repair the side effects of cancer treatment.
Now Peterson and other researchers are expanding their research to study mucositis of the gastrointestinal tract, another troublesome side effect of radiation and chemotherapy.
“Mucosal injury to the mouth may be similar to mucosal injury of the GI tract during high-dose cancer therapy, including chemotherapy,” says Peterson.
Symptoms of GI tract injury include nausea, vomiting and fatigue.
“We are looking at the molecular basis of the oral and GI symptoms to see if there may be common causative mechanisms,” Peterson says. “This line of research could open the door for novel therapies for the constellation of symptoms in oncology patients.”

