|
This is an archived article.
For the latest news, go to the Advance Homepage
For more archives, go to the Advance Archive/Search Page. |
||
|
Pharmacy Students' Findings Stir
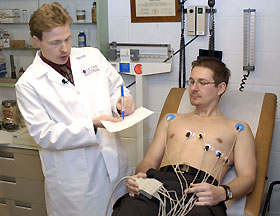
For the past four years, Michael White has required his pharmacy students to investigate an herbal supplement. He never imagined the stir it would cause when he chose Metabolife 356, the nation's number one selling weight-loss supplement, for last year's research project.
The study, which showed that the drug, containing the controversial ingredient ephedra, affects both blood pressure and heartbeat, drew media attention nationwide. Metabolife 356 contains ephedra and 17 other ingredients. White, an associate professor, chose it for study because of its popularity and because any new information revealed by the study might have a significant impact on public health. "Ephedra, also known by its Chinese name ma huang, had been linked to many case reports of people experiencing arrhythmia and stroke," says White, whose expertise is arrhythmia - alteration in the rhythm of the heartbeat - and cardiac pharmacology. "But until our study, no one knew what - if any - effect Metabolife 356 would have on heart rhythm and blood pressure." His students, Angeliki Karapanos and Agnes Krudysz, fifth-year Pharm.D. students doing independent research, wrote a protocol, collected the data and then analyzed the results under the direction of White and with the assistance of pharmacy fellow Brian McBride. The research team recruited 15 healthy, young adults for the study. On one day, the study subjects received half the recommended maximum dosage, and another day they were given a placebo. The patient's blood pressure and electrocardiogram measurements (EKG) were taken immediately before taking a pill, and then at intervals of one, three, and five hours later. The results, presented at the American Heart Association meeting last November, caught the attention of editors of the prestigious Journal of the American Medical Association, the Food and Drug Administration, and dozens of newspapers and TV stations across the country. "Our data showed troubling results in terms of both blood pressure and in the electrical signals that control heartbeat," says McBride. Blood pressure increased on average by 5 percent, he says. But more troubling was what happened to heart rhythm. Over 53 percent of the study subjects experienced an increase of 24 milliseconds in their QTc interval levels, a measure of time that occurs during the electrical impulses generating a heartbeat. Altering the electrical impulses increases the chances for a heart rhythm disturbance. "During this arrhythmia, instead of the normal heartbeat," explains White, "the electrical impulses can cause the heart to quiver and not pump blood effectively." Since the study sample was small and the product contains ingredients other than ephedra, the researchers are not ready to finger ephedra as the primary cause of the changes in heart rhythm and pressure. White and McBride agree, however, that they would recommend that patients stop using Metabolife 356 and other ephedra-containing products. In a month or so, consumers may not have much of a choice. Just days before the UConn study was published in the Jan. 14 issue of the Journal of the American Medical Association, the FDA announced it was taking steps to ban ephedra from the marketplace because of its links to heart attack, stroke, and sudden death. "We know from conversations we've had with the FDA that our study was one of the pieces of evidence it considered while making its decision," says White. He applauds the FDA's unusual action of banning an herbal product. In 1994, Congress passed a law restricting the FDA's authority to regulate herbal supplements, but White believes they need more oversight. Prescription drugs are required to undergo rigorous testing to prove they are safe before the FDA allows them in the marketplace. "In the eyes of the government, pharmaceutical companies are guilty until proven innocent," he says. "But herbal companies are innocent until proven guilty, because they are allowed on store shelves until the FDA has collected enough evidence of their danger. White is hoping many more small studies like his are conducted. He says such studies would yield valuable information about herbal products and their potential risks. Meantime, some of his students are now investigating the ephedra-free version of Metabolife. |